STRUCTURED WATER – What is it?
Water is simple by design, consisting of only two atoms, hydrogen and oxygen. Yet it is one of the most complex of all substances on the earth. It is also the most abundant. Indeed, your own body is composed of almost 70% water by volume! Water is nature’s best solvent, meaning that more substances will dissolve into water than any other substance on the earth.
| WHERE DOES FRESH WATER COME FROM? |
Water must be constantly cleansing and renewing itself. It accomplishes this feat through what is known as the hydrologic cycle. This is an interplay between the two physical actions of evaporation (gaseous) and condensation (liquid), as can be seen depicted in the diagram below:
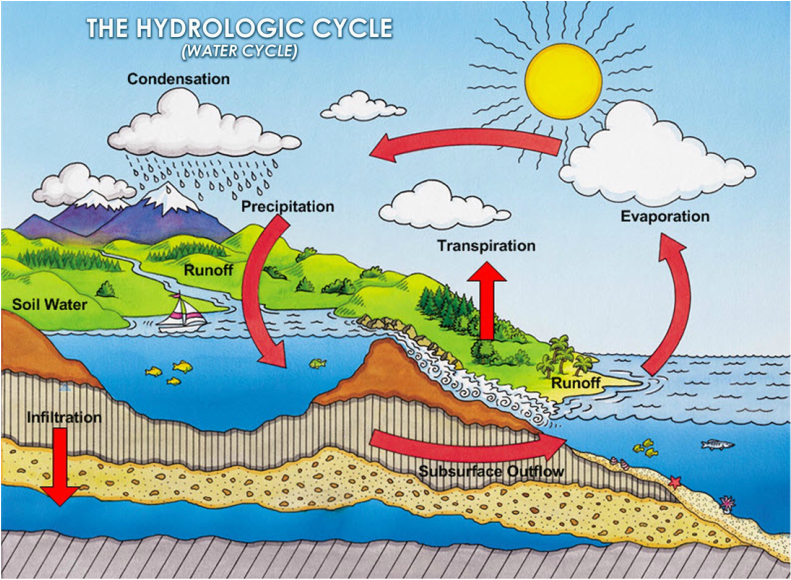
Water is a dynamic medium, continually changing from liquid, to solid, to gas. As it cycles from its gaseous phase in the atmosphere to its liquid and solid forms on the earth, it provides life for the entire earth. The total amount of water on the earth and in the atmosphere has been calculated to be between 1.3 and 1.4 billion km3 (cubic kilometers). That’s a lot of water!
As stated above, the total volume of water never changes – it merely recycles. Three quarters of the surface of the earth is covered by oceans, comprising 97.2% of the total water volume on earth. 2.15% is in the form of ice and 0.001% is included in the atmosphere, which envelopes the earth. Of all the water that continually cycles and recycles on the earth’s surface, only a small percentage is actually available for our use as fresh water.
Usable water includes underground aquifers and above-ground rivers, lakes, streams and marshes, comprising only 0.65% of the total water on the earth. Water must continually cleanse and renew itself through the hydrologic cycle spoken of above. It evaporates from the oceans, seas, lakes and rivers and moves through the atmosphere traveling on wind currents. As the cycle continues, vapor in the atmosphere ultimately changes form and falls to the earth as rain or snow – ending up in rivers and oceans to begin the cycle all over again.
In nature, water movement is an indicator of energy and purity. Water that is moving is generally better than the stagnant water since it contains considerable more amounts of oxygen and minerals.
| WATER’S IMPORTANCE IN ALL LIVING THINGS |
About 70% of the adult human body is composed of water, and there are a hundred times as many water molecules than the total amount of all other molecules in the body put together. 80% to over 90% of our blood and 75% of muscle tissue is comprised of water, and water makes up about 66% of the weight of our cells. As for the water inside the human body; ideally, 60% is found inside the cells (intracellular), and the remaining 40% is situated outside the cells (extracellular).
On a smaller scale than the earth’s hydrologic cycle, but in a similar manner, water circulates through all living organisms: plants, animals and humans; and they all utilize water for very important and specific reasons. In the living organism, water delivers nutrients and oxygen and discharges metabolic wastes. The amount of water that goes into any organism is the same amount that is expelled, and the cycle does not stop as long as the organism is alive and healthy.
In order to live, ideally you must drink between one to three liters of water every day because the water is involved in thousands of bodily functions. The saliva you use to begin digestion and to swallow is composed mostly of water. The movement of your muscles is only possible because they are mostly water, and because they receive their instructions via nerve impulses which are transmitted in water. As humans, we can actually survive for many days or even weeks without food, but if we go without water for even just three or four days we will suffer some very dire consequences.
Water acts as a solvent, a solute, and a reactant, and that is VERY important. Water is actually considered by many to be the universal solvent for life and is referred to by Nobel Laureate A. Szent-Gyorgy as “the matrix of life”. Water serves as the solvent for sodium chloride (salt) and other vital substances. It also serves as the solvent for the electrolytes and nutrients needed by the cells, as well as the solvent to carry waste material away from the cells. It also helps to transport the red blood cells needed to carry oxygen to the cells. With cells bathed in the interstitial fluid, the mechanical functions of osmotic pressure and diffusion supply the needed water itself to the cells. When more complex mechanisms control the transport of molecules across the membranes into and out of cells, the presence of water as the surrounding medium and solvent is essential.
| THE MOLECULAR STRUCTURE OF WATER – A CHEMISTRY LESSON |
To understand water’s important role in life, it helps to understand water itself. As stated above, water is composed of only two atoms – hydrogen and oxygen. Atoms consist of a nucleus which contains both positive protons and neutral neutrons. The nucleus is also surrounded by one or more negatively charged electrons in orbit around them. These orbits are always is a state of flux as they try to gain or lose electrons in order to become stable. Atoms are only satisfied or “happy” if they have a full outer electron shell, which means they will gain or lose electrons in order to complete their outer shell. In this case, the first electron shell of hydrogen can accommodate up to two electrons. When the first electron shell is complete it is stable and less likely to react.
As you can see from the simple diagram below, the hydrogen atom contains only one electron. This leaves one vacant spot in its electron shell. In order for the outer shell of the hydrogen atom to be complete or “happy” it would need to acquire one more electron. Oxygen, unlike hydrogen, can hold up to eight electrons in its outer shell to become stable; therefore, in order for the oxygen atom to be complete it would need to acquire two more electrons.

In the case of the water molecule, hydrogen atoms share their one electron with the oxygen atom. This stabilizes the outer shell of the hydrogen atom. However, the oxygen atom is still unstable since it’s still lacking one electron. But, when a second hydrogen atom bonds with the oxygen atom, filling its outer shell with eight electrons, everyone is “happy”. Whenever two or more atoms share electrons to form a bond these bonds are called covalent bonds, and are one of the strongest bonds in nature.
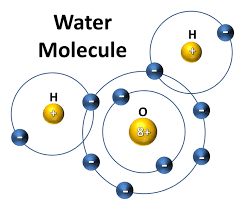
As seen in the diagram below, when the hydrogen and oxygen atoms come together to form the water molecule, there are a total of 10 electrons. One pair in the oxygen’s inner shell, two lone pairs in the outer electrons, and the two pairs shared with the hydrogen atoms.
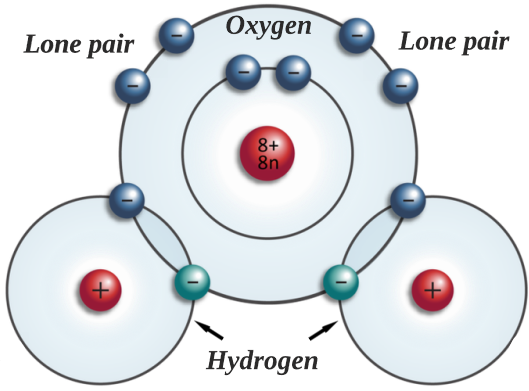
While each water molecule is, as a whole, electrically neutral, they are also polar. Due to the location of the lone electrons on one end and the hydrogen atoms on the other, there are partial positive and partial negative poles. In other words, the area where the lone electrons are located is partially negative whereas the area where the hydrogen atoms are bonded it is partially positive.
Water molecules gain a triangular shape as the result of how hydrogen atoms are bonded to oxygen atoms. As shown in the diagram below, the red sphere is the oxygen atom and the black spheres are the two hydrogen atoms. The rods between the oxygen and hydrogen atoms represent molecular hydrogen bonds.
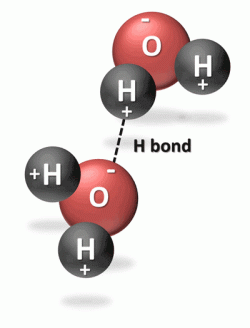
Many of the physical and chemical properties of water are due to its structure. Rather than the hydrogen atoms bonding directly opposite to one another on the oxygen atom, the atoms in the water molecule are arranged with the two hydrogen-oxygen bonds at an angle of about 105°.
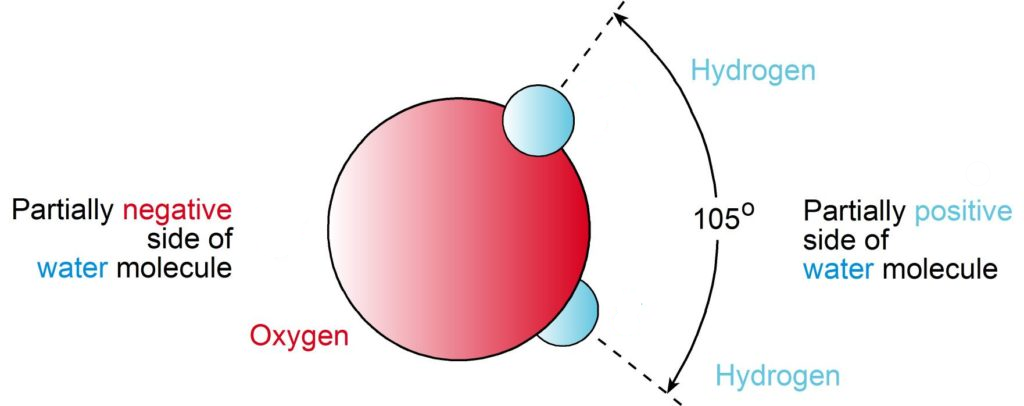
From the pictures and explanations above, you can see that the water molecule, as whole, has a positive and a negative side. As we all know, opposites attract. This is the same with water molecules. Under optimum conditions, the water molecules attract one another in precise geometric patterns called “clusters,” with the positive end of one water molecule lined up with the negative end of another water molecule. This attraction is called the electric dipole.
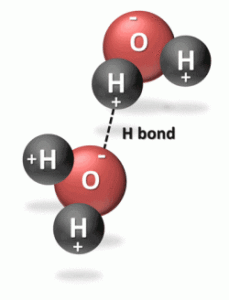
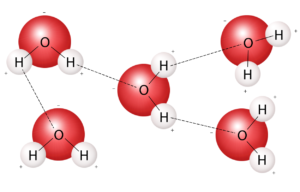
 The electric dipole gives rise to attractions between neighboring opposite ends of water molecules. Each oxygen atom is attracted to nearby hydrogen atoms. Such bonding is referred to as hydrogen bonding. Although considerably weaker than the covalent bonds holding the water molecules together, hydrogen bonding is strong enough to keep water liquid at ordinary temperatures.
The electric dipole gives rise to attractions between neighboring opposite ends of water molecules. Each oxygen atom is attracted to nearby hydrogen atoms. Such bonding is referred to as hydrogen bonding. Although considerably weaker than the covalent bonds holding the water molecules together, hydrogen bonding is strong enough to keep water liquid at ordinary temperatures.
Various other properties of water are due to these hydrogen bonds. One of these properties is its high specific heat, meaning it takes more energy to increase the temperature of 1 gram of water 1 degree Celsius, compared to other substances.
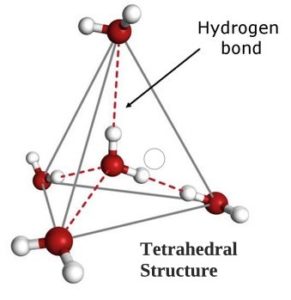
However, as the temperature of water is lowered, clusters of molecules begin to form through hydrogen bonding, each oxygen atom can bond with four hydrogen atoms, and simultaneously each water molecule can have up to four hydrogen bonds. This, in turn, creates a tetrahedral (or four sided) arrangement.
| STRUCTURED WATER |
 Every farmer knows that rainwater is better for their crops and livestock than irrigation water. The reason is simple. Rainwater falling from the sky is refreshed, energized and transformed by the rays of the sun, the swirling motion of the wind, the electrical charge of lightning, and by the natural design of the atmosphere itself.
Every farmer knows that rainwater is better for their crops and livestock than irrigation water. The reason is simple. Rainwater falling from the sky is refreshed, energized and transformed by the rays of the sun, the swirling motion of the wind, the electrical charge of lightning, and by the natural design of the atmosphere itself.
Crystal Blue Water units enhance water in much the same way. Their unique design accomplishes one of nature’s greatest miracles of regeneration and renewal by mimicking the natural vortex motion of water as it falls to the earth as rain and tumbles along a mountain stream, creating a measurable increase in water’s ability to hydrate and nourish plant and animal tissues, penetrate soils, and conserve water. This results in healthier and more resilient crops and livestock.